sp3 carbon–fluorine bond activation in 2,2-difluorohomoallylic alcohols via nucleophilic 5-endo-trig cyclisation: synthesis of 3-fluorinated furan derivatives†
Abstract
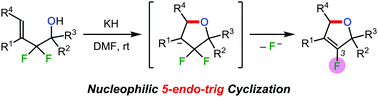
Nucleophilic 5-endo-trig cyclisation was achieved in 2,2-difluorohomoallylic alcohols. Upon treatment with potassium hydride, 2,2-difluorohomoallylic alcohols underwent an intramolecular SN2′-type reaction to afford 3-fluoro-2,5-dihydrofurans in high yields. In addition, the oxidation of these dihydrofurans formed 4-fluorofuran-2(5H)-ones. Thus, ring-fluorinated furan derivatives were efficiently obtained via allylic sp3 carbon–fluorine bond cleavage.


 Please wait while we load your content...
Please wait while we load your content...