Copper-catalyzed decarboxylative propargylation/hydroamination reactions: access to C3 β-ketoester-functionalized indoles†
Abstract
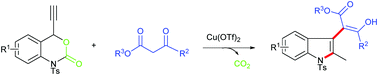
A copper-catalyzed reaction of ethynyl benzoxazinanones with readily accessible β-ketoesters via a decarboxylative propargylation/hydroamination sequence has been developed. This protocol furnished a diverse range of C3 β-ketoester-functionalized indoles in good to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...