Enhancement and control of the selectivity in light-driven ketone versus water reduction using aminopyridine cobalt complexes†
Abstract
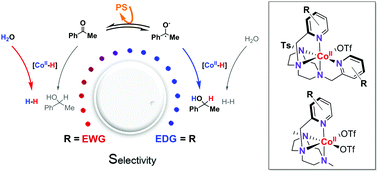
Cobalt(II) complexes with the general structure [CoII(OTf)(Y,XPy2Tstacn)](OTf) (1R, where Y,XPy2Tstacn is 1,4-di(p-Y,m-X-picolyl)-7-R-1,4,7-triazacyclononane; 1H, 1CO2Et, 1DMM) and [CoII(OTf)2(Y,XPyMetacn)] (2R, where Y,XPyMetacn is 1-(p-Y,m-X-picolyl)-7,4-di-methyl-1,4,7-triazacyclononane; 2CO2Et, 2Cl, 2H, 2DMM, 2NMe2) were active in both light-driven acetophenone (3a) and water reduction. Competition studies show that aromatic ketone/water reduction selectivity ranks from 0.2 to 8.0. Nevertheless, considering the concentrations of water and ketone in catalysis (ratio H2O/3a ∼ 2000) the highest selectivity obtained is greater than 15 000. The selectivity correlates well with the CoI/II redox potential within the same cobalt catalyst series (span 240 mV (1R) and 290 mV (2R)), with electron donating ligands favoring ketone reduction over H2 evolution. Based on this finding, the operative mechanism for the reduction of aromatic ketones is consistent with a single electron transfer (SET) followed by a hydrogen atom transfer (HAT) mechanism. This new insight will be a guide to develop selective catalytic systems to produce fine solar chemicals in water.


 Please wait while we load your content...
Please wait while we load your content...