Enhancing the stability of CsPbBr3 nanocrystals by sequential surface adsorption of S2− and metal ions†
Abstract
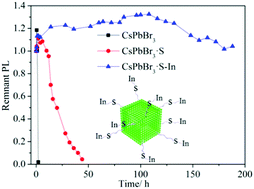
A sequential surface adsorption method for improving the photostability of perovskite nanocrystals (NCs) at room temperature was proposed. Firstly, S2− was decorated on the surface of CsPbBr3 NCs by adding didodecyl dimethylammonium sulfide (S2−-DDA+), and then In3+ ions, which diffused from the salt powder of In(Ac)3, were slowly adsorbed by the pre-loaded S2−. Through this process, the photoluminescence quantum yield of the CsPbBr3 NCs increases from 57% to 80%, and their photostability, thermal stability, and colloidal stability were all drastically improved. The resultant CsPbBr3·S–In NCs remained stable for 188 h under strong LED light illumination (450 nm and 175 mW cm−2), while pristine CsPbBr3 NCs totally quenched in 2 h, which shows the great potential of CsPbBr3·S–In NCs in lighting and display applications. We also demonstrated that this method could be extended to many other metal salts to form stable perovskite·S–X (X = Ni, Mn and Zn) nanocrystals.


 Please wait while we load your content...
Please wait while we load your content...