Highly Lewis acidic cationic alkaline earth metal complexes†
Abstract
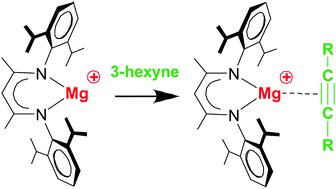
Cationic Lewis base-free β-diketiminate (BDI) complexes of Mg and Ca have been isolated as their B(C6F5)4− salts. The cation (BDI)Mg+ shows an extraordinarily strong Lewis acidity that can compete with strong Lewis acids like B(C6F5)3 and (BDI)AlMe+. Its highly electrophilic nature is exemplified by isolation of an 3-hexyne adduct.
- This article is part of the themed collection: Celebrating our 2020 Prize and Award winners


 Please wait while we load your content...
Please wait while we load your content...