Frustrated Lewis pairs in ionic liquids and molecular solvents – a neutron scattering and NMR study of encounter complexes†
Abstract
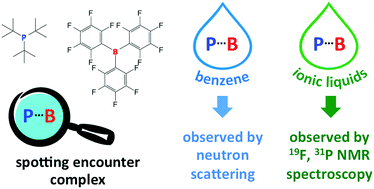
The presence of the weakly-associated encounter complex in the model frustrated Lewis pair solution (FLP): tris(tert-butyl)phosphine (P(tBu)3) and tris(pentafluorophenyl)borane (BCF) in benzene, was confirmed via P⋯B correlation analysis from neutron scattering data. On average, ca. 5% of dissolved FLP components were in the associated state. NMR spectra of the FLP in benzene gave no evidence of such association, in agreement with earlier reports and the transient nature of the encounter complex. In contrast, the corresponding FLP solution in the ionic liquid, 1-decyl-3-methylimidazolium bistriflamide, [C10mim][NTf2], generated NMR signals that can be attributed to formation of encounter complexes involving over 20% of the dissolved species. The low diffusivity characteristics of ionic liquids is suggested to enhance high populations of encounter complex. The FLP in the ionic liquid solution retained its ability to split hydrogen.
- This article is part of the themed collection: Ionic Liquids in the Synthesis, Fabrication, and Utilization of Materials and Devices


 Please wait while we load your content...
Please wait while we load your content...