Selective one- and two-electron reductions of a haloborane enabled by a π-withdrawing carbene ligand†
Abstract
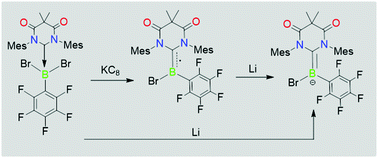
A carbene-stabilised neutral boryl radical and a boryl anion are isolated via selective one- and two-electron reduction of a diamidocarbene (DAC) adduct of dibromo(pentafluorophenyl)borane. Both the radical and the anion have been characterised by various spectroscopic techniques in solution, while the structures have been ascertained by single-crystal X-ray diffraction. In contrast, the reduction of the analogous cyclic (alkyl)(amino) carbene (CAAC) adduct yields a C–H activation product.


 Please wait while we load your content...
Please wait while we load your content...