Mild rhodium(iii)-catalyzed intramolecular annulation of benzamides with allylic alcohols to access azepinone derivatives†
Abstract
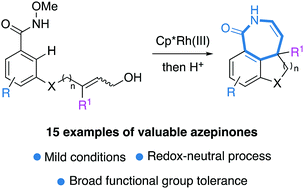
Azepinone derivatives are important frameworks of several natural products and bioactive compounds. They are synthetized using a Rh(III)-catalyzed intramolecular annulation of benzamide-tethered allylic alcohols. The reaction requires mild conditions at room temperature and affords diversely substituted azepinones bearing a quaternary carbon.


 Please wait while we load your content...
Please wait while we load your content...