Electrolyte-gated transistors based on phenyl-C61-butyric acid methyl ester (PCBM) films: bridging redox properties, charge carrier transport and device performance†
Abstract
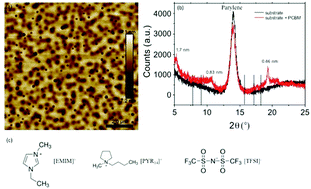
The n-type organic semiconductor phenyl-C61-butyric acid methyl ester (PCBM), a soluble fullerene derivative well investigated for organic solar cells and transistors, can undergo several successive reversible, diffusion-controlled, one-electron reduction processes. We exploited such processes to shed light on the correlation between electron transfer properties, ionic and electronic transport as well as device performance in ionic liquid (IL)-gated transistors. Two ILs were considered, based on bis(trifluoromethylsulfonyl)imide [TFSI] as the anion and 1-ethyl-3-methylimidazolium [EMIM] or 1-butyl-1-methylpyrrolidinium [PYR14] as the cation. The aromatic structure of [EMIM] and its lower steric hindrance with respect to [PYR14] favor a 3D (bulk) electrochemical doping. As opposed to this, for [PYR14] the doping seems to be 2D (surface-confined). If the n-doping of the PCBM is pursued beyond the first electrochemical process, the transistor current vs. gate-source voltage plots in [PYR14][TFSI] feature a maximum that points to the presence of finite windows of high conductivity in IL-gated PCBM transistors.


 Please wait while we load your content...
Please wait while we load your content...