Homoallylglycine residues are superior precursors to orthogonally modified thioether containing polypeptides†
Abstract
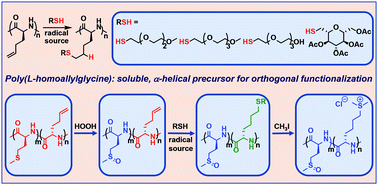
Homoallylglycine N-carboxyanhydride, Hag NCA, monomers were synthesized and used to prepare polypeptides containing Hag segments with controllable lengths of up to 245 repeats. Poly(L-homoallylglycine), GHA, was found to adopt an α-helical conformation, which provided good solubility in organic solvents and allowed high yield functionalization of its alkene side-chains via radical promoted addition of thiols. The conformations of these derivatives were shown to be switchable between α-helical and disordered states in aqueous media using thioether alkylation or oxidation reactions. Incorporation of GHA segments into block copolymers with poly(L-methionine), M, segments provided a means to orthogonally modify thioether side-chains different ways in separate copolypeptide domains. This approach allows preparation of functional polypeptides containing discrete domains of oxidized and alkylated thioether containing residues, where chain conformation and functionality of each domain can be independently modified.


 Please wait while we load your content...
Please wait while we load your content...