Hetero-assembly of a dual β-amyloid variant peptide system†
Abstract
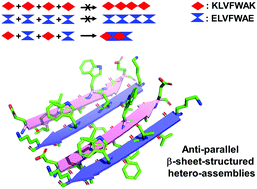
Self-assembly of amyloid polypeptides (1) imparts biological effects depending on the size in over 20 amyloid diseases and (2) produces useful yet relatively untapped biomaterials. Unfortunately, our understanding of amyloid polypeptides, as related to biomedical implications and biomaterial applications, is limited by their self-assembling nature. In this study, we report the creation of a dual peptide system, where a pair of β-amyloid (Aβ) variants are not self-assembled but hetero-assembled in the presence of their assembly partners. We provide evidence that the resulting hetero-assemblies share molecular, structural and morphological similarities with typical amyloid self-assemblies formed by a single polypeptide (e.g., Aβ). We anticipate that our dual peptide system may readily be adapted for precise control of amyloid assembly, for the study of size-dependent neurotoxicity and precise fabrication of amyloid biomaterials.


 Please wait while we load your content...
Please wait while we load your content...