Transition-metal-free C–H amidation and chlorination: synthesis of N/N′-mono-substituted imidazopyridin-2-ones from N-pyridyl-N-hydroxylamine intermediates†
Abstract
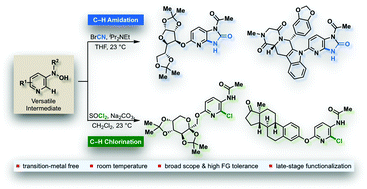
Non-symmetric 1,3-substituted imidazopyridin-2-ones are a common structural scaffold found among many biologically active molecules. Herein we report an efficient, mild, and transition-metal free C–H amidation strategy to access such a pyrido-fused cyclic urea framework in good yields and with a broad functional group tolerance.
- This article is part of the themed collection: 2018 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...