Origins of halogen effects in bioorthogonal sydnone cycloadditions†
Abstract
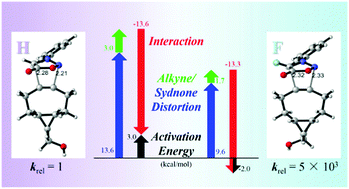
Halogen substituents increase sydnone cycloaddition reactivities substantially. Fluoro-sydnones are superior to bromo- and chloro-sydnones, and can achieve extremely high second-order rate constants with strained alkynes. Computational studies have revealed the fluorine substituent increases the reactivity of sydnone mainly by lowering its distortion energy.


 Please wait while we load your content...
Please wait while we load your content...