Tandem cyclization of o-alkynylanilines with isocyanides triggered by intramolecular nucleopalladation: access to heterocyclic fused 2-aminoquinolines†
Abstract
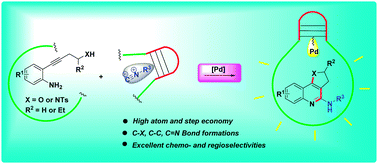
Herein, a novel strategy for the synthesis of various heterocyclic fused 2-aminoquinolines via palladium-catalyzed tandem cyclization of o-alkynylanilines with isocyanides has been developed. This process includes trans-oxy/aminopalladation, isocyanide insertion, elimination and 1,3-hydrogen migration. Besides high atom and step economy, this method shows good functional group compatibility with excellent chemo- and regioselectivities under mild reaction conditions.
- This article is part of the themed collection: 2018 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...