Ring-expanded N-heterocyclic carbenes as ligands in iron-catalysed cross-coupling reactions of arylmagnesium reagents and aryl chlorides†
Abstract
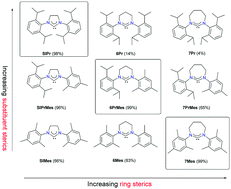
The structure–activity relationship of expanded-ring N-heterocyclic carbenes (NHCs) in the iron-catalysed Kumada aryl–aryl coupling reaction was explored. This was achieved by comparing the catalytic performance of Fe-NHC catalysts generated in situ containing NHCs that differ in steric bulk. In particular, the influences of ring sizes (5–8) and N-aryl substituents were explored in terms of spectroscopic and structural features, which affect their %Vbur values. The three best performing ligands were found on a diagonal of a 5 × 4 structural matrix revealing an optimal steric bulk and significant influences of subtle steric variations on the catalytic activities.


 Please wait while we load your content...
Please wait while we load your content...