Evidence for anion-binding of all-cis hexafluorocyclohexane in solution and solid state†
Abstract
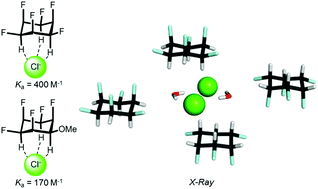
We report a solution NMR and X-ray crystallographic study on the anion affinity of all-cis 1,2,3,4,5,6-hexafluorocyclohexane, which has only recently become synthetically accessible. Our results suggest that the interaction exhibits preferential 1 : 1 stoichiometry, while its strength is only moderate (e.g. Ka = 400 M−1 in acetone for Cl−) and depends mainly on the size of the anion and the dielectric constant of the solvent.


 Please wait while we load your content...
Please wait while we load your content...