Highly enantioselective transfer hydrogenation of racemic α-substituted β-keto sulfonamides via dynamic kinetic resolution†
Abstract
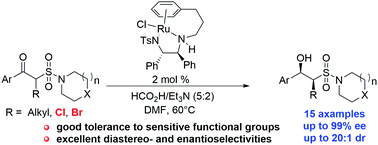
Highly enantioselective transfer hydrogenation of β-keto sulfonamides was developed via dynamic kinetic resolution using a chiral Ru(II) catalyst with an azeotropic solution of HCO2H/Et3N as a hydrogen donor, affording α-substituted β-hydroxyl sulfonamides in good yields with excellent diastereo- and enantioselectivities. This method is featured with mild conditions, easy operation, and a broad substrate scope, which make it possible to find wide applications in the synthesis of natural products and biologically active compounds containing the α-substituted β-hydroxyl sulfonamide core.


 Please wait while we load your content...
Please wait while we load your content...