Metal-free site selective cross-coupling of pyridines with secondary phosphine chalcogenides using acylacetylenes as oxidants†
Abstract
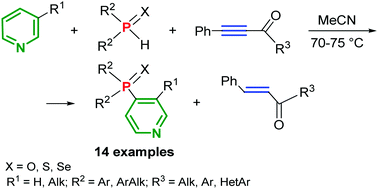
Pyridines undergo site selective cross-coupling with secondary phosphine chalcogenides (oxides, sulfides, and selenides) in the presence of acylphenylacetylenes under metal-free mild conditions (70–75 °C, MeCN) to afford 4-chalcogenophosphoryl pyridines in up to 71% yield. In this new type of SNHAr reaction acylacetylenes act as oxidants, being stereoselectively reduced to the corresponding olefins of the E-configuration.


 Please wait while we load your content...
Please wait while we load your content...