Recognition of shorter and longer trimethyllysine analogues by epigenetic reader proteins†
Abstract
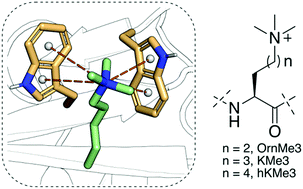
Histone Nε-lysine methylation is a widespread posttranslational modification that is specifically recognised by a diverse class of Nε-methyllysine binding reader proteins. Combined thermodynamic data, molecular dynamics simulations, and quantum chemical studies reveal that reader proteins efficiently bind trimethylornithine and trimethylhomolysine, the simplest Nε-trimethyllysine analogues that differ in the length of the side chain.


 Please wait while we load your content...
Please wait while we load your content...