Hydrogen-motivated electrolysis of sodium carbonate with extremely low cell voltage†
Abstract
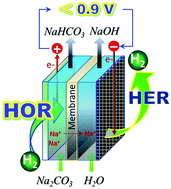
Hydrogen-motivated electrolysis of Na2CO3 for energy-saving production of NaOH and CO2/NaHCO3 is realized by the hydrogen oxidation reaction to insert proton into anolyte and the hydrogen evolution reaction to extract proton out of catholyte. Electrolytic voltage at 100 mA cm−2 is as low as 0.88 V; this voltage is only 35% of the voltage used in the traditional electrolysis.


 Please wait while we load your content...
Please wait while we load your content...