Design of bifunctional chiral phenanthroline ligand with Lewis basic site for palladium-catalyzed asymmetric allylic substitution†
Abstract
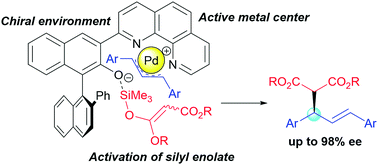
Conceptually new bifunctional chiral ligands were developed. The axially chiral N,N-bidentate phenanthroline ligand (S)-1 was found to be effective for Pd-catalyzed asymmetric allylic substitution of allyl acetate and dialkyl malonate. The intramolecular Lewis basic group from the hydroxybinaphthyl structure of (S)-1 played a pivotal role in the high reactivity and enantioselectivity.


 Please wait while we load your content...
Please wait while we load your content...