Copper-catalyzed decarboxylative cyclization via tandem C–P and C–N bond formation: access to 2-phosphorylmethyl indoles†
Abstract
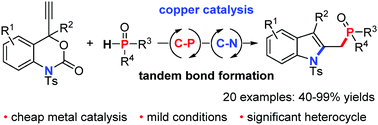
A novel copper-catalyzed decarboxylative cyclization of ethynyl benzoxazinanones with P(O)H compounds has been developed. This protocol leads to a series of 2-phosphorylmethyl indoles with high efficiency and selectivity (up to 99% yield) through tandem C–P/C–N bond formation under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...