Gold-catalyzed N,O-functionalization of 1,4-diyn-3-ols with N-hydroxyanilines to form highly functionalized pyrrole derivatives†
Abstract
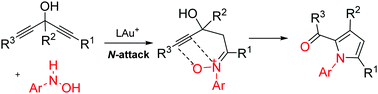
This work describes new N,O-functionalization of 1,4-diyn-3-ols with N-hydroxyanilines to yield highly functionalized pyrrole derivatives. In a postulated mechanism, N-hydroxyaniline attacks the more electron-rich alkynes via regioselective N-attack to form unstable ketone-derived nitrones that react with their tethered alkynes via an intramolecular oxygen-transfer to form α-oxo gold carbenes. This new method is applicable to a short synthesis of a bioactive molecule, a PDE4 inhibitor.


 Please wait while we load your content...
Please wait while we load your content...