Chiral triazolylidene-Pd-PEPPSI: synthesis, characterization, and application in asymmetric Suzuki–Miyaura cross-coupling†
Abstract
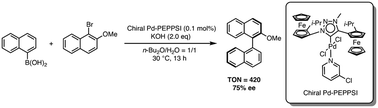
A chiral triazolylidene-Pd-PEPPSI (PEPPSI = pyridine, enhanced, precatalyst, preparation, stabilization, initiation) complex with ferrocene-based planar chirality has been synthesized and characterized. Investigation of the electronic and steric nature of this complex revealed its powerful electron-donating ability (TEP 2044 cm−1) and high steric bulk (%Vbur = 42.2). These unique properties allow the complex to exhibit very high catalytic activity (TON = 420) for asymmetric Suzuki–Miyaura cross-coupling, providing the coupling product with good enantioselectivity (75% ee).


 Please wait while we load your content...
Please wait while we load your content...