Iron-catalyzed C(sp3)–H functionalization of N,N-dimethylanilines with isocyanides†
Abstract
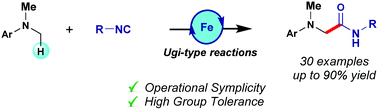
An efficient ligand-free Fe-catalyzed oxidative Ugi-type reaction toward the assembly of α-amino amides and short peptides is described. The reaction proceeds through the α-C(sp3)–H oxidation of N,N-dimethylanilines and further nucleophilic attack of the resulting iminium species by isocyanides. Additive screening showed that judicious choice of the carboxylic acid could lead to the formation of α-amino imides via a 3-component reaction. The process occurs with operational simplicity and is compatible with a variety of sensitive functional groups.


 Please wait while we load your content...
Please wait while we load your content...