One-dimensional hydrogen bonding networks of bis-hydroxylated diamantane formed inside double-walled carbon nanotubes†
Abstract
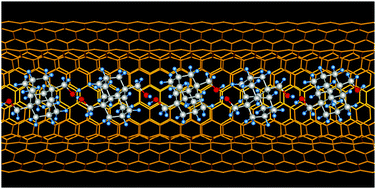
1,6-Bis(hydroxymethyl)diamantane spontaneously aligns inside double-walled carbon nanotubes. The encapsulated molecules form a one-dimensional network within the double-walled carbon nanotubes through hydrogen bonding that leads to a highly dense filling as compared to unfunctionalized diamantane. Improving the encapsulation yields of precursors via functionalization is crucial to prepare novel one-dimensional materials.


 Please wait while we load your content...
Please wait while we load your content...