Dialumination of unsaturated species with a reactive bis(cyclopentadienyl) dialane†
Abstract
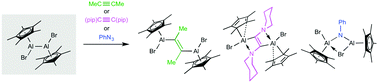
A new bis(cyclopentadienyl) dialane is prepared, which shows controlled, selective dialumination reactions with a conventional alkyne, an electron-rich alkyne, and an azide. The reactions provide structurally diverse products, featuring a range of aluminium coordination numbers, cyclopentadienyl binding modes, and cyclic motifs. The variable nature of the bonding in the Cp*Al units allows a range of binding modes depending on the electronic requirements of the Al atom and provides new possibilities to the chemistry of dialanes, as demonstrated by the isolation of a double internal Lewis adduct with “ring-slipped” Cp* rings in this work.
- This article is part of the themed collection: Philip Power at 65: an icon of organometallic chemistry


 Please wait while we load your content...
Please wait while we load your content...