Enantioselective cooperative proton-transfer catalysis using chiral ammonium phosphates†
Abstract
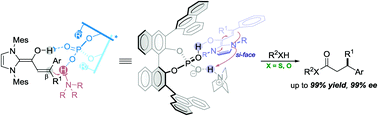
Chiral phosphorate anions are shown to be highly enantioselective templates for proton-transfer catalysis. A salt generated in situ from a bridgehead amine and a BINOL-derived chiral phosphoric acid serves as an effective proton-shuttle that exhibits remarkable enantioselectivity in a bioinspired, triple co-operative catalysis involving an achiral NHC. Thioesters with a β-chiral center can be prepared in a single step from substituted cinnamaldehyde derivatives, with up to 99% yield and 99% ee. Heteroaryl groups are well tolerated in these reactions, despite the presence of basic sites.


 Please wait while we load your content...
Please wait while we load your content...