Electrochemical initiation of electron-catalyzed phenanthridine synthesis by trifluoromethylation of isonitriles†
Abstract
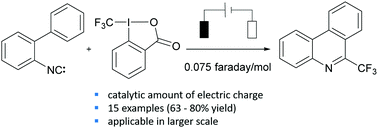
The electron-catalyzed formation of phenanthridines starting from isonitriles initiated by an electrochemical reduction of the Togni reagent is presented. The required number of faradays per mole of starting material and the respective yields clearly show the catalytic character of the electron in this reaction. The mechanism is supported by cyclic voltammetry experiments.
- This article is part of the themed collection: Celebrating our 2019 Prize and Award winners


 Please wait while we load your content...
Please wait while we load your content...