Cyclic amino(carboranyl) silylene: synthesis, structure and reactivity†
Abstract
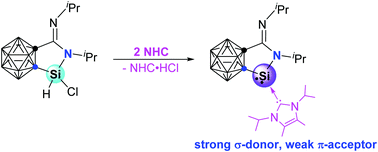
A carbene-stabilized cyclic amino(carboranyl) silylene has been prepared from the reaction of cyclic amino(carboranyl) chlorosilane with N-heterocyclic carbene via HCl elimination. Its structure has been characterized by single-crystal X-ray analysis and DFT calculations. It is a very strong σ donor and relatively poor π acceptor. It can form a Lewis acid–base adduct with borane and undergo cycloaddition reactions with unsaturated molecules such as diphenylacetylene and benzophenone.


 Please wait while we load your content...
Please wait while we load your content...