Ionic liquids with trichloride anions for oxidative dissolution of metals and alloys†
Abstract
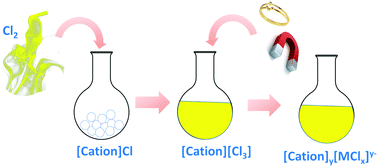
Ionic liquids (ILs) with trichloride anions ([Cl3]−) combined with different cations were synthesised by bringing chlorine gas into contact with the corresponding chloride (Cl−) ILs at room temperature. These trichloride ILs safely store chlorine and are useful as oxidising agents for dissolution of various metals and alloys under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...