Chemoselective triazole-phosphonamidate conjugates suitable for photorelease†
Abstract
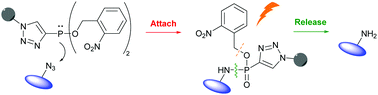
Herein, we describe a new method for the conjugation of azide-containing target compounds that can be readily released as amines by irradiation with near UV light. This concept is based on a two-step protocol employing the chemoselective CuAAC and Staudinger-phosphonite reactions to deliver photo-cleavable phosphonamidate conjugates in high yields starting from 2-nitrobenzyl substituted phosphonites.


 Please wait while we load your content...
Please wait while we load your content...