Organic semiconductor perylenetetracarboxylic diimide (PTCDI) electrodes for electrocatalytic reduction of oxygen to hydrogen peroxide†
Abstract
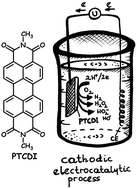
Hydrogen peroxide is one of the most important industrial chemicals and there is great demand for the production of H2O2 using more sustainable and environmentally benign methods. We show electrochemical production of H2O2 by the reduction of O2, enabled by an organic semiconductor catalyst, N,N′-dimethyl perylenetetracarboxylic diimide (PTCDI). We make PTCDI cathodes that are capable of stable and reusable operation in aqueous electrolytes in a pH range of 1–13 with a catalytic figure of merit as high as 26 kg H2O2 per g catalyst per h. These performance and stability open new avenues for organic small molecule semiconductors as electrocatalysts.


 Please wait while we load your content...
Please wait while we load your content...