Conformational control of nonplanar free base porphyrins: towards bifunctional catalysts of tunable basicity†
Abstract
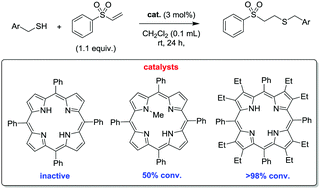
For the first time, free base and N-methylated porphyrins have been utilized as bifunctional organocatalysts in Michael additions and it was found that distortion of the macrocycle is a vital prerequisite for their catalytic activity. Conformational design has been used to tailor the properties of nonplanar porphyrins with regards to availability of the N–H units for hydrogen bonding (distortion-dependent hydrogen bonding) and the basicity of the heterocyclic groups. NMR spectroscopic- and catalyst screening studies provided insight into the likely mode of catalyst action. This unprecedented use of free base and N-substituted porphyrins as organocatalysts opens a new functional role for porphyrins.


 Please wait while we load your content...
Please wait while we load your content...