Enantioselective total synthesis of (−)-kainic acid and (+)-acromelic acid C via Rh(i)-catalyzed asymmetric enyne cycloisomerization†
Abstract
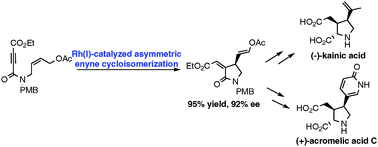
A diversity-oriented synthetic strategy was developed for the total synthesis of kainoid amino acids, which led to the enantioselective synthesis of (−)-kainic acid and the first total synthesis of (+)-acromelic acid C. Rh(I)-catalyzed asymmetric enyne cycloisomerization served as the key reaction in this strategy for the rapid construction of highly functionalized lactam, and the resulting vinyl acetate moiety was further utilized as a versatile building block for the installation of both isopropylidene and 2-pyridone units existing in natural kainoids.


 Please wait while we load your content...
Please wait while we load your content...