Treatment of dry eye by intracanalicular injection of a thermosensitive chitosan-based hydrogel: evaluation of biosafety and availability
Abstract
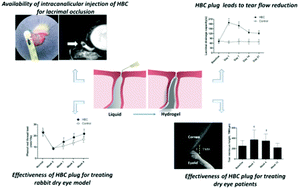
Chitosan has been increasingly considered for the design of implant materials in the field of translational medicine. However, implant properties addressing the complexity of the desired tissue still need to be developed. The focus of this study lies in the evaluation of a thermosensitive chitosan-based hydrogel for intracanalicular injection. Hydroxybutyl chitosan (HBC) solution was prepared, and its cytocompatibility was investigated by the CCK-8 assay using primary human corneal epithelial cells (HCEpiCs). Minimal cytotoxicity was seen in cultures with the HBC-extracting solution at a concentration of 0.2 g ml−1 for up to 72 h incubation. The biocompatibility and effectiveness, based on both a rabbit model and a human pilot study, were evaluated anatomically and functionally. The flow flux significantly decreased after HBC injection, with 76.9% of the flow flux occurring 10 min after HBC injection. Tear secretion significantly improved in the rabbit model. The density of PAS-positive cells gradually increased in the animal model. Various clinical indicators, which include the ocular surface disease index (OSDI) and tear break up time, have been improved greatly. Thermosensitivity promotes greater suitability for HBC intracanalicular injection to obstruct the lacrimal drainage system at body temperature. These results demonstrate that the thermosensitive chitosan-based hydrogel is suitable as a liquid plug for tear flow blockage and thus represents a promising candidate for translational medicine.


 Please wait while we load your content...
Please wait while we load your content...