Magnetic delivery of Fe3O4@polydopamine nanoparticle-loaded natural killer cells suggest a promising anticancer treatment†
Abstract
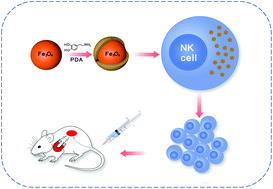
Natural killer (NK)-cell-based immunotherapy has been reported to have promising prospects in the treatment of non-small cell lung cancer, one of the most common malignancies in the world. It has been proven that higher the NK cell infiltration into the tumor, the better is the curative effect. Therefore, it would be beneficial to develop a method that increases NK cell recruitment and infiltration into the tumor site. The purpose of this study was to establish an immune-cell delivery system for clear lung cancer cells based on magnetic nanoparticle (NP)-labeled NK cells that can be accumulated at the tumor site by placing a tiny external magnetic device inside animals. We developed superparamagnetic iron oxide NPs consisting of a magnetic Fe3O4 core and a shell of polydopamine (PDA) for magnetic targeting therapy. Fe3O4@PDA NPs possess favorable physiological stability and biocompatibility that facilitate their active uptake by NK cells. The biology of NK cells was not affected by the presence of NPs. In vitro and in vivo studies showed that Fe3O4@PDA NP-labeled NK cells significantly inhibited tumor growth and reduced the expression of Ki-67 and increased the apoptosis of A549 cancer cells. H&E staining showed Fe3O4@PDA NP-labeled NK cells, under a magnetic field, had higher intra-tumoral iron density and increased accumulation of CD56+ NK cells. Our results suggest that Fe3O4@PDA NPs are a promising magnetic nanomaterial that can manipulate immune cells, thereby inhibiting tumor growth.


 Please wait while we load your content...
Please wait while we load your content...