Microcapsules as assay compartments formed through layer-by-layer deposition†
Abstract
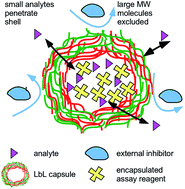
We investigate the potential of using microcapsules, generated through layer-by-layer assembly, as reaction compartments for bioassays. A streptavidin–biotin and an esterase-fluorescein diacetate (FDA) assay were employed for proof-of-concept. Streptavidin was incorporated into the microcapsules via two methods: (i) physical adsorption onto MnCO3 microcrystal templates or (ii) co-precipitation with CaCO3 to form mixed microcrystals. The streptavidin-loaded template microparticles were then coated with five bi-layers of polyelectrolytes (polyallylamine hydrochloride/polystyrene sulfonate) (PAH/PSS)5 and the templated cores were dissolved using EDTA to form polyelectrolyte microcapsules loaded with streptavidin. Streptavidin loading of around 77 mg streptavidin per mol of CaCO3 was achieved using the co-precipitation method, compared to 5.1 mg streptavidin per mol of MnCO3 using physical adsorption onto the manganese carbonate crystal surface. The microencapsulated streptavidin was allowed to bind to an analyte which was able to freely diffuse through the polyelectrolyte shell from the aqueous media. Biotin-4-fluorescein was added to the streptavidin-loaded microcapsules, with streptavidin-free capsules serving as the control sample. It was found that both types of microcapsules exhibited a similar fluorescence signal intensity, likely due to non-specific binding of biotin-4-fluorescein to charged groups on the polyelectrolyte shell. Therefore, an alternative esterase-FDA assay was investigated as fluorescence is only produced when FDA penetrates the capsule shell and reacts with the esterase enzyme inside which leads to its hydrolysis. Esterase was loaded into the LbL capsules templated on CaCO3via co-precipitation. FDA was added to both the esterase-loaded capsules and esterase-free capsules. It was found that the capsules containing esterase fluoresced, while the esterase-free capsules did not. This indicates that the hydrolysis reaction of FDA with esterase occurred inside the LbL microcapsules. These experiments demonstrate the potential of compartmentalised assays to be carried out inside microcapsules which could be applied in complex matrices or in multiplexing experiments, wherein different barcoded microcapsules contain different reagents to provide independent readouts without interference from larger biomolecules present in the surrounding media.


 Please wait while we load your content...
Please wait while we load your content...