Facile preparation of Ag nanoparticles using uric acid and their applications in colorimetric detection and catalysis†
Abstract
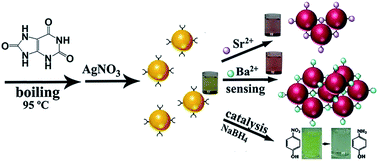
In this study, we have reported a facile, cost-effective method for the synthesis of silver nanoparticles (Ag NPs) using uric acid (UA) as the reducing agent. Techniques such as UV-visible spectroscopy, transmission electron microscopy and X-ray spectroscopy were performed to characterize the generated UA–Ag NPs. UA–Ag NPs exhibited excellent specificity towards Ba2+ and Sr2+, which could be traced by UV-visible spectra and the naked eye; we also obtained the lowest detection limits of 52.1 nM and 27.5 nM for Ba2+ and Sr2+, respectively. The proposed method provided sensitive and rapid detection of Ba2+ and Sr2+ at the nM level in water samples, and the values were much lower than the U. S. EPA standard for the maximal limits of these metal ions in drinking water. UA–Ag NPs could be employed as a colorimetric probe to assay Ba2+ and Sr2+ in lake water, indicating their potential applicability for detection in real samples. As far as we know, this is the first attempt for the colorimetric detection of Sr2+ by noble metal nanoparticles. Furthermore, UA–Ag NPs exhibited excellent potential for catalyzing the reduction of nitrophenols and potassium ferricyanide.
- This article is part of the themed collection: Analytical Methods Recent HOT articles


 Please wait while we load your content...
Please wait while we load your content...