Comparison of poly(styrene-divinylbenzene)-based monolithic and bead-based methodologies used in NANOFLOW LCMS for proteomic studies†
Abstract
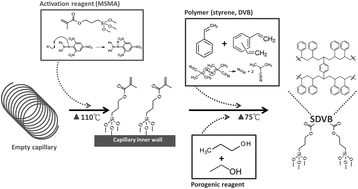
Nanoflow liquid chromatography coupled with electrospray tandem mass spectrometry (nanoLC-ESI-MS/MS) is a powerful tool in proteomics analysis. The optimum conditions for preparing and the performance of a high efficiency polymeric column were compared with those of micro-particle-filled capillary columns, including a totally porous silica C18 column and a HALO® fused core C18 column. Tryptic peptides were used as model compounds for evaluating the performance of three in-house fabricated columns. After optimization, a monolithic capillary column was prepared by the in situ polymerization of styrene and divinylbenzene (PS–DVB) within a 50 μm i.d. fused silica capillary using 1-propanol as the porogen. These continuous unitary porous structures are more robust and efficient compared with bead-based columns. A meter level PS–DVB column could substantially reduce the co-elution of peptides and suppress ion suppression, thus permitting the total ion current signal to be significantly enhanced. These three columns were comparable for the routine identification of peptides. For glycopeptides, the monolithic PS–DVB column gave the highest separation efficiency and a total of 20 N-linked glycopeptides could be identified in a tryptic digest of fetuin and bevacizumab (Avastin). The results indicate that a PS–DVB column has great potential for separating hydrophilic peptides. The novel monolithic media described represent a promising addition to the stationary phase used in capillary columns for proteome research.


 Please wait while we load your content...
Please wait while we load your content...