Clinical analysis and quantitation of MB-102, a novel fluorescence tracer agent, in human plasma†
Abstract
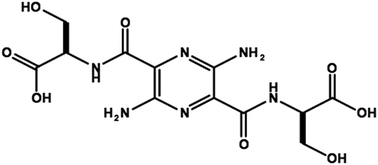
A rapid analytical method for the quantitation of MB-102 (a fluorescent tracer agent) in human plasma using HPLC with fluorescence detection has been developed and validated. This new plasma sample preparation method, 1/100 direct dilution with phosphate buffered saline instead of typical protein precipitation, improves both robustness and assay efficiency by eliminating the need for sample drying and the need for an internal standard. The validation results show that the new method is robust, specific, precise, accurate, and has a wide linear dynamic range (0.4 ng mL−1 to 400 ng mL−1) with good recovery. The results from 59 clinical study subjects analyzed by both methods yield an excellent correlation with a slope of 1.015 and an R2 of 0.994. Sample preparation using direct dilution is easier and faster than the protein precipitation method, and is excellent for determining the MB-102 content in human plasma. The concentration of MB-102 in plasma as a function of time is used to calculate the glomerular filtration rate using a standard pharmacokinetic model.

 Please wait while we load your content...
Please wait while we load your content...