Confocal Raman micro-spectral evidence and physicochemical evaluation of triamterene salts†
Abstract
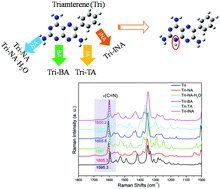
Discrimination of active pharmaceutical ingredients (APIs) existing as neutral molecules or salts is essential and complicated. However, the discrimination of pharmaceutical salts by confocal Raman micro-spectroscopy remains relatively poorly understood. In this paper, four new salts of triamterene (Tri) cocrystallized with nicotinic acid (NA), benzoic acid (BA), p-toluenesulfonic acid (TA), or isonicotinic acid (INA) were prepared and characterized comprehensively by powder X-ray diffraction (PXRD), differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), polarized light microscopy (PLM), and dynamic vapor sorption (DVS). Ionized pteridine is identified by marker peaks in the confocal Raman micro-spectra that are characteristic of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N. The single crystal structures of Tri-NA·H2O and Tri-TA further demonstrate that a proton transfers from the carboxylic group of NA or TA to the pyrimidine N1 atom of Tri and their salts formation take place. The intrinsic dissolution rate (IDR) and apparent equilibrium solubility of these four salts are improved compared to the pure Tri component, especially for Tri-BA. This study provides a valuable insight into pharmaceutical salt discrimination by vibrational spectroscopy and presents that the combination of Tri with an acid can be a possible and promising alternative formulation of Tri.
N. The single crystal structures of Tri-NA·H2O and Tri-TA further demonstrate that a proton transfers from the carboxylic group of NA or TA to the pyrimidine N1 atom of Tri and their salts formation take place. The intrinsic dissolution rate (IDR) and apparent equilibrium solubility of these four salts are improved compared to the pure Tri component, especially for Tri-BA. This study provides a valuable insight into pharmaceutical salt discrimination by vibrational spectroscopy and presents that the combination of Tri with an acid can be a possible and promising alternative formulation of Tri.


 Please wait while we load your content...
Please wait while we load your content...