Systematic analysis of various ionic liquids by attenuated total reflectance spectroscopy (145–450 nm) and quantum chemical calculations†
Abstract
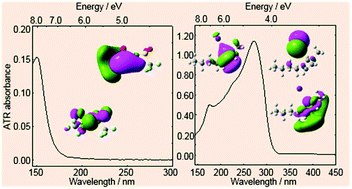
Despite providing rich information on electronic states, the far-ultraviolet (FUV, <200 nm) and deep-ultraviolet (DUV, <300 nm) absorption spectra of ionic liquids (ILs) are difficult to obtain without saturation due to very strong analyte absorbance. Herein, FUV-DUV spectra of selected ILs were systematically and easily recorded using an attenuated total reflectance spectrometer and rationalized based on quantum chemical calculations. ILs containing pyrrolidinium or ammonium cations and fluorine-containing anions exhibited weak absorbance below 200 nm that could not be measured by conventional UV-Vis spectroscopy, whereas the corresponding imidazolium-based ILs showed distinct absorption bands that could be reproduced by single-cation-model calculations. On the other hand, imidazolium-based ILs with halide anions showed characteristic charge transfer (CT)-related absorbances. Thus, the above spectroscopic investigations contribute to a fundamental understanding of the electronic processes (e.g., intramolecular excitations and CT transitions) and molecular designs used in electrochemical devices.
- This article is part of the themed collection: Analyst Recent HOT articles


 Please wait while we load your content...
Please wait while we load your content...