Molecularly imprinted polymer nanoparticle-based assay (MINA): application for fumonisin B1 determination†
Abstract
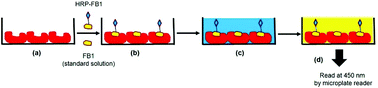
The enzyme-linked immunosorbent assay (ELISA) has been used as a standard tool for monitoring food and animal feed contamination from the carcinogenic fumonisin B1 (FB1). Unfortunately, ELISA is not always efficient due to the instability of the antibody and enzyme components in the immunoassay, the presence of natural enzyme inhibitors in the samples and the high levels of non-specific protein binding. Additionally, the production of antibodies for ELISA can be time-consuming and costly, due to the involvement of animals in the manufacturing process. To overcome these limiting factors, a molecularly imprinted nanoparticle based assay (MINA) has been developed, where the molecularly imprinted nanoparticles (nanoMIPs) replace the primary antibody used in a competitive ELISA. Herein, computational modelling was used to design the nanoMIPs by selecting monomers that specifically interact with FB1. The affinity of the monomers to FB1 was verified by measuring their binding in affinity chromatography experiments. The nanoMIPs were produced by solid phase synthesis and the results showed that nanoMIPs had a hydrodynamic diameter of around 249 ± 29 nm. The assay tested in model samples is highly selective and does not show cross-reactivity with other mycotoxins such as fumonisin B2 (FB2), aflatoxin B1 (AFB1), citrinin (CTT), zearalenone (ZEA), and deoxynivalenol (DON). The MINA allows the detection of FB1 in the concentration range of 10 pM–10 nM with a detection limit of 1.9 pM and a recovery of 108.13–113.76%.


 Please wait while we load your content...
Please wait while we load your content...