Perturbation of microRNA signalling by doxorubicin in spermatogonial, Leydig and Sertoli cell lines in vitro†
Abstract
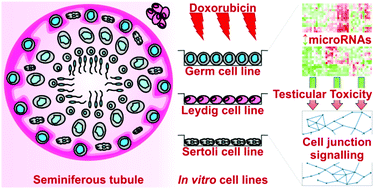
We have previously shown that in addition to its widely recognised cardiotoxicity, the chemotherapeutic doxorubicin (DOX) is able to induce transcriptional, microRNA (miRNA) and DNA methylation changes in the mouse testis. These changes perturb pathways involved in stress/cell death and survival and testicular function and lead to germ cell loss and reproductive organ damage. Here, we further investigated the differential miRNA expression induced by DOX in mouse spermatogonial (GC1), Leydig (TM3) and Sertoli (TM4) cell lines in vitro. We began by performing cell cycle analysis of the three mouse testicular cell lines to evaluate their sensitivity to DOX and thus select suitable doses for miRNA profiling. In keeping with our in vivo data, the spermatogonial cell line was the most sensitive, and the Sertoli cell line the most resistant to DOX-induced cell cycle arrest. We then further demonstrated that each cell line has a distinct miRNA profile, which is perturbed upon treatment with DOX. Pathway analysis identified changes in the miRNA-mediated regulation of specialised signalling at germ–Sertoli and Sertoli–Sertoli cell junctions following treatment with DOX. Amongst the most significant disease categories associated with DOX-induced miRNA expression were organismal injury and abnormalities, and reproductive system disease. This suggests that miRNAs play significant roles in both normal testicular function and DOX-induced testicular toxicity. Comparison of our in vitro and in vivo data highlights that in vitro cell models can provide valuable mechanistic information, which may also help facilitate the development of biomarkers of testicular toxicity and high-throughput in vitro screening methods to identify potential testicular toxicants.


 Please wait while we load your content...
Please wait while we load your content...