Isoindigo benzodifurandione based conjugated polymers for high performance organic field-effect transistors†
Abstract
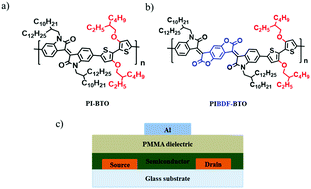
We report isoindigo based copolymers, poly(isoindigo benzodifurandione-bithiophene-alkoxyl) (PIBDF-BTO) and poly(isoindigo-bithiophene-alkoxyl) (PI-BTO) for high performance organic field-effect transistors (OFETs). PIBDF-BTO was synthesized by introducing strong electron withdrawing BDF into the acceptor isoindigo unit of PI-BTO to deepen the polymer highest occupied molecular orbital and the lowest unoccupied molecular orbital. This modification provided better polymer chain crystallinity and connectivity in thin film through increased intermolecular interactions and larger co-planarity in PIBDF-BTO. X-ray diffraction and atomic force microscopy showed higher crystallinity and well-arranged nano-fibril morphological PIBDF-BTO film compared with PI-BTO film. PIBDF-BTO OFETs showed much higher field effect mobility with well-balanced ambipolar transport with hole and electron mobilities of 0.27 and 0.22 cm2 V−1 s−1 compared to PI-BTO OFETs with hole mobility 0.004 cm2 V−1 s−1 and very low electron mobility. PIBDF-BTO also showed better hole and electron injection properties due to the narrower band gap (0.97 eV) compared to PI-BTO (1.35 eV). PI-BTO and PIBDF-BTO OFETs were operationally stable with negligible threshold voltage shift after continuous cycling measurements.


 Please wait while we load your content...
Please wait while we load your content...