Synthesis and thermoelectric properties of 2- and 2,8-substituted tetrathiotetracenes†
Abstract
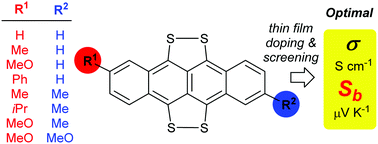
Reaction of elemental sulfur with 2-R1 and 2,8-R1,R2-substituted tetracenes (2) in refluxing DMF affords 5,6,11,12 tetrathiotetracenes (1) in good yields (74–99%) for a range of substituents where R1,R2 are: H,H (a); Me,H (b); MeO,H (c); Ph,H (d); Me,Me (e), iPr,Me (f, iPr = iso-propyl, CHMe2), Me,MeO (g); MeO,MeO (h). The reaction rate is limited only by the solubility of the tetracene (2); 2g–h being both the least soluble and slowest reacting. At partial conversion recovered single crystalline 2g led to its X-ray structure determination. Vacuum deposited (substrate deposition temperature 300 K, pressure 7 × 10−6 mbar, source temperature 500 K) thin films from 1 (of initial 88–99% purity) show final electrical conductivities, σ(in plane) from 1.40 × 10−5 S cm−1 (1g) to 3.74 × 10−4 S cm−1 (1b) for the resultant near pristine films; while 1d proved too involatile to be effectively sublimed under these conditions. In comparison, initially 95% pure TTT (1a) based films show σ(in-plane) = 4.33 × 10−5 S cm−1. The purities of 1a–h are highly upgraded during sublimation. Well defined micro-crystallites showing blade, needle or mossy like habits are observed in the films. The Seebeck coefficients (Sb) of the prepared 1 range from 374 (1c) to 900 (1f) μV K−1 (vs. 855 μV K−1 for identically prepared 95% pure TTT, 1a). Doping of films of 1f (R1 = iPr, R2 = Me) with iodine produces optimal p-type behaviour: σ(in-plane) = 7.00 × 10−2 S cm−1, Sb = 175 μV K−1. The latter's power factor (PF) at 0.33 μW m−1 K−2 is more than 500 times that of the equivalent I2-doped TTT films (1a, R1 = R2 = H), previously regarded as the optimal material for thin film thermoelectric devices using acene radical cation motifs.


 Please wait while we load your content...
Please wait while we load your content...