Enhancing the performance of non-fullerene organic solar cells via end group engineering of fused-ring electron acceptors†
Abstract
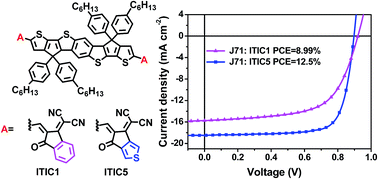
A fused heptacyclic electron acceptor, ITIC5, based on a benzodi(cyclopentadithiophene) core flanked by thiophene-fused termini, is designed, synthesized, and compared with its benzene-fused analogue, ITIC1. ITIC5 with thiophene-fused termini exhibits a narrower optical band gap, stronger and redshifted absorption, and higher electron mobility than ITIC1. The active layer consisting of a wide-bandgap polymer donor J71 and ITIC5 exhibits a smaller acceptor domain, stronger crystallinity, and higher and more balanced mobilities than its J71:ITIC1 counterpart, contributing to efficient exciton dissociation and charge transport. J71:ITIC5 based organic solar cells exhibit a high fill factor of 75.5% and a champion power conversion efficiency of 12.5%, a nearly 40% boost in efficiency with respect to the ITIC1-based control device, suggesting that the thiophene-fused end group has great potential for constructing high-performance fused-ring electron acceptors.


 Please wait while we load your content...
Please wait while we load your content...