N-Alkyl substituted 1H-benzimidazoles as improved n-type dopants for a naphthalene-diimide based copolymer†
Abstract
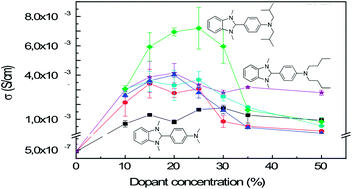
Doped polymer semiconductors are actively studied for opto- and micro-electronic applications including thermoelectric generators, where a high electrical conductivity is a key factor. In general, n-type doping is more challenging to achieve than p-type doping. Here we study n-type doping of a commonly used electron transporting naphthalene-diimide bithiophene copolymer with a series of air-stable and solution-processable benzimidazole dopants. To understand the role of dopant structure on miscibility and the resulting conductivity, benzimidazoles with different linear and branched alkyl substituents were synthesized, and their doping efficacy compared through combined morphological, electrical and thermoelectric characterization. We observe a clear dependence of the nature of the alkyl substituent on dopant intercalation into the semicrystalline morphology. By increasing the length or the steric hindrance of the alkyl substituents, the miscibility between dopant and copolymer is enhanced leading to optimized electrical conductivity.


 Please wait while we load your content...
Please wait while we load your content...