Nonaqueous arylated quinone catholytes for lithium–organic flow batteries†
Abstract
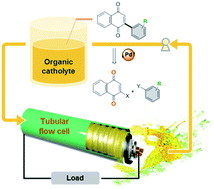
Chemically modified organic redox couples have the advantages of tunable redox properties, high solubility, environmental benignity, and cost effectiveness. Inspired by nature, a series of quinone derivatives bearing electron-donating methoxy or electron-withdrawing trifluoromethyl groups are synthesized in moderate to high yields by Pd-catalyzed Suzuki cross-coupling reactions. This study utilizes the synthetic quinones as redox-active organic molecules for nonaqueous lithium–organic flow batteries. The aryl moiety incorporated quinone scaffolds show enhanced electrochemical stability and rate capability. The nonaqueous catholyte, 2-phenyl-1,4-naphthoquinone, reaches a cell voltage of ∼2.6 V and a specific capacity of 196 mA h g−1, while the discharge capacity is retained at ∼92% for 150 cycles. Moreover, the tubular lithium–organic flow battery system features stable cycle performance under a continuous circulation without clogging-associated intermittency flow.


 Please wait while we load your content...
Please wait while we load your content...